 |
| HARSHAW DEPARTMENTS
(Incomplete)
NOTE: CLICK ON ANY PICTURE TO ENLARGE
The Harshaw Chemical Company was a diversified specialty chemical company. Not only did it supply a large number of specialty chemicals such as fluoride containing compounds, nickel salts and glycerine but many other products. Harshaw was recognized as a prime producer of catalysts, pigments, electroplating processes, synthetic optical crystals and ceramic products as well as many organic and inorganic compounds. Below is a listing of some of the plants and the products they produced. Below are photographs of some of the offices/plants as well as individual departments. |
| At the right is an aerial view of the main offices and a portion of the original research and analytical laboratories located on E. 97th Street in Cleveland, OH until moving to Beachwood, OH in 1982. As can be seen, the original school structure had been converted into general offices as well as laboratories, the latter known as the Laurel Labs. | 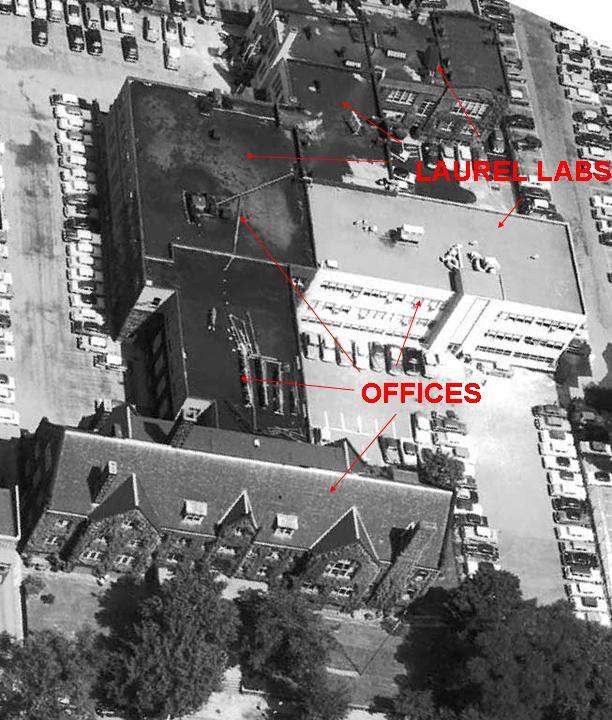 |
|  | At the left is the Harvard-Denison plant located in the "flats" area of downtown Cleveland, OH. This facility produced fluorides, such as; anhydrous hydrofluoric acid and fluoboric acid; nickel salts, such as nickel sulfates, chlorides, sulfamates and fluoborates; electroplating additives, for nickel, copper, chromium and other electroplating processes. During WWII, this facility was converted to produce uranium hexafluoride used in the manufacture of the atomic bomb used to end the war. |
| METAL FINISHING DEPARTMENT
| |
|  | Circa 1973. This photo was taken by the Harshaw Advertising Department to show the extent of the Metal Finishing Department. Shown are the MF Sales and Technical Service groups as well support departments, such as the Analytical and Order/Entry Departments. |
| The booth of the Metal Finishing Department displayed at the American Electroplaters Society Convention held in Cleveland, OH in 1972. |  |
|  | Some of the promotional "give-aways" of the Metal Finishing Department.
Top left - Poker Matchbook; Top right - BBQ Cleaner;
Center left - paperweight; Center right - pocket knife/fingernail file;
Bottom - math converter using many of the units used in electroplating |
| INDUSTRIAL CHEMICAL DEPARTMENT | |
| | As mentioned above, the ICD products were mainly produced at the Harvard-Denison Plant in Cleveland, OH. The Company was originally founded by reselling various chemicals such as lard oil, aqua ammonia and turpentine followed later by the manufacture of glycerine, nickel anodes and nickel salts, the latter products used in the electroplating industry. This grew into the manufacturing of manganese oxide, tin oxide, chromium oxides and various other metallic oxides some of which were used in the ceramic industry.Antimony oxide was also manufactured for the enameling industry and later as a fire retardant. The Company grew into the manufacture of hydrofluoric acid and then sodium and ammonium fluorides. Eventually Harshaw grew into the role of a leading supplier of fluorides and nickel chemicals. During WWII, Harshaw operated a super secret facility for the manufacture of uranium hexafluoride for the Manhattan Project which was subsequently refined to produce the material for the first atomic bomb. After the war, Harshaw was the world's leading supplier of nickel sulfate and associated nickel chemicals and also continued its dominance of fluoride chemicals. The Company also entered into the manufacture of paint driers, pigments, glass enamels and catalysts to name but a few. |
| At right, an ICD tanker truck used for transporting anhydrous hydrofluoric acid. |  |
|  | The ICD Cleveland sales group circa 1973. |
| ICD Sales meeting held at Quail Hollow Resort in Concord, OH in June 1981. |  |
|  | ICD Sales meeting also held at Quail Hollow Resort in September 1982. |
| CRYSTAL and ELECTRONICS DEPARTMENT
Many people made Harshaw a leader in this industry throughout its existence. In paricular, two distinguished gentlemen who graciously narrated much of the background and substance for this section, Carl F. Swinehart, PhD, and David A. Hammond, PhD, took time in late 1999 and late 2003 to provide much of this information. |  |
| |
Carl Swinehart began his career with Harshaw Chemical in 1932 and consulted for the company and its successors into the late 1990's. David Hammond began his career with Harshaw in 1950 and consulted weekly until 2000 with Bicron, a survivor of the Harshaw heritage. Both earned their doctoral degrees from Western Reserve University in Cleveland, OHio.
To bring the accomplishments of these two outstanding scientists into perspective. not only was their loyalty and longevity noteworthy, but Dr. Swinwhart is the holder of 29 US Patents (includint three in 2001) and Dr. Hammond holds another five. The US Patent issued to Dr. Swinehart in 1947 for the fluourine cell is among the most important of these as it allowed for the production of uranium hexafluoride used in the making of the atomic bomb. (see alo the Industrial Chemical Department above).
Others who have contributed and otherwise distinguished themselves with Harshaw and left us are Elmer C. Stewart (deceased) and Dr. Robert Hofstadter, Dr. James Schulman, John Cameron and Herb Attix.
Elmer Stewart was a graduate chemist and was Vice president and General Manager of the Crystal and Electronic Department for some twenty years. Under Stewart's management and leadership, Harshaw became the worldwide leader in the development and supply of commercially available scintillation radiation detectors, namely with sodium iodide (thallium activated) and others, thermoluminescence (or TL) materials such as lithium fluoride, calcium fluoride and related thermoluminesence dosimetry (or TLD) instruments and systems. The Crystal and Electronics Department often engaged the expertise of Nobel Laurate Dr. Robert Hofstadter of Princeton and Stanford Universities in developing the scintillation detector program and business. Similarly, the CED also called on outstanding consultants such as Dr. James Schulman of the Naval Research Laboratory (NRL), John Cameron of the University of Wisconsin and Herb Attix of both the NRL and the University of Wisconsin in developing the successful TLD program.
Harshaw entered crystal growing in the 1930's with LiF crystals as a substitute for fused quartz with optical resonance at around 3 microns for the analysis of organic compounds containing CH2. LiF has excellent vacuum ultraviolet performance from 3 microns down to 1 micron. Optical spectrometry using LiF revolutionized the analysis and synthesis of many organic compounds. Pure sodium chloride (NaCl) was developed beginning in 1937 for similar applications. Harshaw grew synthetic sapphire and ruby from pressed aluminum oxide (Al2O3). Hot-pressed magnesium fluoride (MGF2) is the infrared detector material used in heat-seeking missles, smart weapons and some night vision devices.
In the late 1940's, sodium fluoride doped with thallium (NaI(Tl)) was found to produce scintillation from incident ionizing photon radiations and patented as a scintillation material by Dr. Hofstadter and colleagues. Circa 1950, John Harshaw, son of the founder of the Harshaw Chemical Company, initiated interest in growing NaI(Tl) crystals by the Stockbarger method. Dr. Stockbarger was another consultant to Harshaw for optical crystal growth during previous years.
In 1952, Elmer Stewart, then Sales manager for the C&EP Department, unveiled two freshly canned (NaI(Tl) crystals at the second IEEE Nuclear Science Symposium to loud acclaim and great popularity. Sodium iodide dpoed with thallium was hygroscopic and needed to be hermetically sealed, or canned.
During the remainder of the 1950's, 1960's and even into the 1970's, Harshaw made dramatic improvements in terms of crystal growing including incrystal sizes, crystal quality and configurations for NaI(Tl) and other scintillation, optical and thermoluminescent crystals. For example, by 1970, Harshaw was able to grow NaI(Tl) crystal as large as 32" in diameter and 12" thick.
|
| NaI(Tl) is and has been widely used in imaging cameras for nuclear medicine applications. NaI(Tl) continues to be the most widely used scintillation crystal material in the world. |  |
|  | In the 1970-s, the development of a NaI(Tl) extrusion process, shown at right, enabled a variety of cross sections suitable for minimal blank machining times and higher product yields from the normal right cylinder ingots. |
| In the meantime, Harshaw also developed some other scintillation crystals to meet various customer needs and applications including CsI(Tl), CsI(Na), BaF2, CaF2, LiI, PbF, and a few others. The LiI is used for neutron detection, the BaF2 (pure) is an example of a high atomic number (Z) photon detector and PbF2 is an excellent Cherenkov radiation detector. More recently Bismuth Germanate (BGO) has been developed for some applications. The positive properties of BGO include no need to be canned and it maintains its polish.
Thermoluminescence dosimeters (TLDs), along with complementary TL measuring instrumentation developed by Harshaw, have been used worldwide for some decades for photon, beta and neutron dosimetry.Harshaw also developed silver chloride (AgCl) used by salt-water batteries, which are employed in torpedoes and sonar buoys to detect the presence and motion of submarines. Dr. H.C. Kremers realized that he needed help and experience in rolling “Clago” sheet, AgCl, for the Army Signal Corps. Through channels, Harshaw and the Signal Corps asked the steel company in Lorain, Ohio, for someone with experience in rolling steel that would roll the new material (AgCl).
Harshaw was acquired by Kewaunee Oil Company in 1964. Kewaunee Oil was then acquired by the Gulf Oil Company in 1976. Kaiser Chemical Company next acquired the Harshaw interests in 1982. Engelhard Corporation acquired some of Harshaw in the late 1980s, and existing former Harshaw interests are currently under the management of Saint Gobain, a French consortium.
Many thanks to Morgan Cox for the above history of this Department. | |
| | | | |
|
|
 |
|
